
Crossing boundaries: from the blood-brain barrier to the disciplines of science
By April Cashin-Garbutt
When Viktor Plattner first met visiting scholar Viviana Gradinaru at SWC they spoke about an exciting new blood-brain barrier (BBB) crossing virus in development in her lab at Caltech. Viktor was eager to try out the new adeno-associated virus (AAV) in his neuroscience research in rats. He shared the resulting confocal images with Viviana’s team, which were published in a Nature Communications paper. This initial collaboration led to a pioneering new direction for his research project in the Akrami lab at SWC.
Engineering viruses to cross the blood-brain barrier
For many years, medical researchers have been trying to engineer viruses to cross the blood-brain barrier in an attempt to tackle multiple neurological diseases. At Caltech, scientists have been working on developing AAVs that only target specific cell types in the body, which means they can be used for gene therapy.
Whilst these viruses have huge potential in medical research, by allowing faulty genes in specific cell types to be replaced by healthy genes, they could also have a major impact on foundational neuroscience by allowing scientists to target specific neurons in the brain.
“The reason this is very exciting is because at the moment we have limited tools to image or manipulate neural circuits in rats. There are many transgenic mouse lines that enable scientists to study the neural activity during mouse behaviour very well. However, rats are a completely different species and much more challenging to study without such transgenic tools,” explained Viktor Plattner, SWC Research Fellow.
Crossing the boundaries of science
The team hope that these AAVs could offer a minimally invasive tool to label functionally meaningful networks in rat brains that can be manipulated or imaged in different tasks.
Viktor has been working with Sian Murphy in the SWC’s Neurobiological Research Facility (NRF) to deliver the viruses to the rats in a minimally invasive way. The AAVs are injected systemically, yet they are able to travel into the brain because they can cross the blood-brain barrier.
While the AAVs travel everywhere in the body, they only enter neurons in the brain because they have been detargeted from peripheral organs. The team at SWC are also exploring ways to target specific neuron types: either through engineering the shell of the virus, known as the capsid, to target specific brain cells, or through making the cargo carried by the capsid selective so that even if the virus enters every neuron the gene it carried will only be expressed in specific types. The hope is that these new tools can be combined with existing neuroscience techniques, such as wide-field imaging and optogenetic manipulation to allow scientists to study neural activity in rats.
“Rats are much more social animals compared with mice and they can learn more complex tasks. This means they can be better models for some neurological problems and behaviours that are present in humans and can give us directions to design more insightful psychophysics experiments in humans,” said Viktor Plattner.
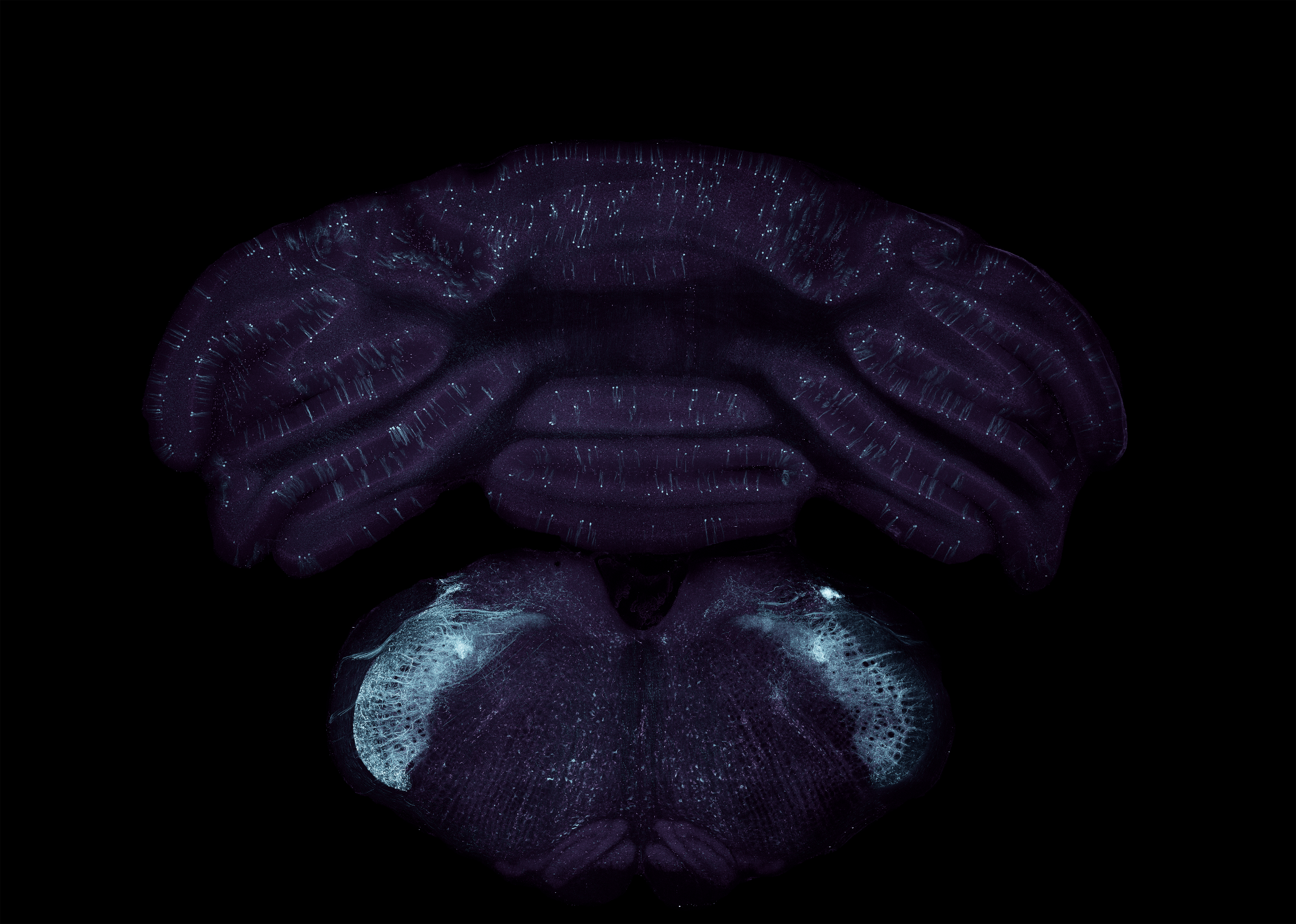
Virally transduced neurons in the cerebellum
Next steps
The Akrami Lab team are working on quantifying the concentration of virus that needs to be injected to transduce a functionally meaningful number of neurons in the brain. They are also exploring how to make this process neuron- and region- specific.
Piotr Nowak, Research Technician in the Viral Vector Core at SWC, is helping the team develop and fine-tune the reproduction of the viruses from Caltech, and Peter Gordon, Jessica Broni-Tabi and Rob Campbell in the Advanced Microscopy Facility at SWC are also providing support to the project.
The next steps are to test whether the AAVs can deliver functional genes into specifically-targeted neurons and express proteins such as GCaMP, a calcium-binding fluorescent protein used for imaging neural activity, and Channel rhodopsin, a light-gated ion channel used for optogenetics.
“If we can show that these functional proteins work in specific-targeted neurons after systemic injection of this virus, then this could be a game-changer for the neuroscience research community,” commented Viktor Plattner.
Find out more
- Read the research paper in Nature Communications: Functional gene delivery to and across brain vasculature of systemic AAVs with endothelial-specific tropism in rodents and broad tropism in primates
- Find out more about research in the Akrami Lab
Banner image shows virally transduced neurons in the hippocampus


